

Overview
Looking at the pictures above, you may wonder what these floating objects are made of. Are they really metallic objects? Yes, they are metallic objects. One is a metallic coin, and the other is a metallic needle. How is it possible that metallic objects can float in water? The Short answer is Surface Tension (ST).
Though the densities of these metals are much greater than the density of water, we can make them float if we carefully place them on the surface.
What makes these metal objects float?
It is the phenomenon called Surface Tension which makes a small metal coin or a needle float. Okay. But what is it? What gives rise to this phenomenon in liquids? Where else can we experience this phenomenon?
Let us explore this phenomenon now. Keep reading.
Cohesive and Adhesive
Forces
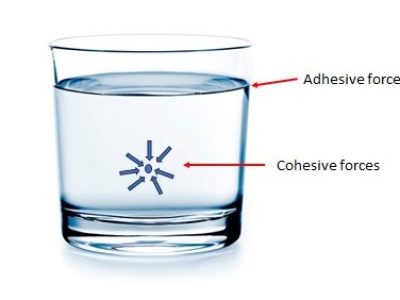
In order to understand ST, we need to explain first what cohesive and adhesive forces are.
Consider a glass of water. We know that it consists of billions and billions of water (H2O) molecules. Each molecule experiences strong intermolecular attraction forces, known as Van de Waals force, from the neighboring molecules because of electrostatic bonding. When these intermolecular attraction forces are between like molecules, they are referred to as cohesive forces.
If these intermolecular attraction forces are between unlike molecules, say between water molecules and glass molecules, then we call them adhesive forces.
Now let us see how these cohesive forces in a liquid result in ST on the surface of a liquid.
Surface Tension
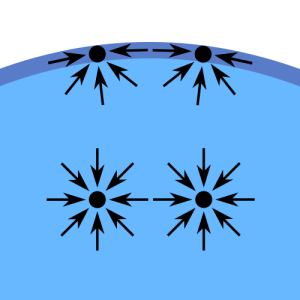
As mentioned above, cohesive forces in a liquid are due to the electrostatic attraction forces between like molecules. The molecules, well inside the liquid, experience equal-cohesive forces from all directions. See picture 4.
As a result, there is no net cohesive force on those molecules. However, that is not the case with molecules on the surface. These molecules on the liquid surface experience net inward cohesive force because there are more attraction forces from the molecules below than the attraction forces from the above as there are no water molecules above the surface. In addition, these surface molecules experience lateral-attractive forces from the molecules on their sides.
These net forces, on the surface molecules, create a thin liquid membrane with tension on it. This tension on the surface of a liquid manifests as surface tension. We need to apply a little force to penetrate this membrane. If an object does not apply enough force to penetrate the membrane (like a light metallic needle), the ST holds the object floating. That is what is happening in the case of light coin too.
ST Examples
Spherical water droplet:

As you can see in the picture 5, a water droplet becomes spherical when it is falling freely. This is because of ST. The ST minimizes the surface area by making it spherical. As you may know, surface area is the least for a sphere for a given volume. Hence a freely falling water droplet turns itself into a perfect sphere because of ST.
Floating Metal Objects

When we carefully place a needle or a light metallic coin on water, they float because their weight is not enough to penetrate the surface membrane. You can see these floating metallic objects in pictures 1, 2 and 6.
Soap and detergents
Why do we use soap or detergent for cleaning? How do they help the cleaning process? When they are dissolved in water, they reduce the ST of water. Consequently, water readily soaks into pores and soiled areas.
Walking on water
Small insects such as water strider can walk on water as their weights are not enough to penetrate the surface membrane formed because of the ST.
Capillary Action
The capillary action, as the video below shows, is the spontaneous flow of fluid in porous material or in narrow tubes. It is caused by the combination of adhesive and cohesive forces; especially when the adhesive forces overpowers the cohesive forces.
Conclusion
Surface tension is a special property of fluids that makes it act like it has a stretchy skin on top. Because of this, light objects like coins or pins can float on water.
Surface tension also helps explain why water drops are round, why soap helps clean better, and how tiny insects can walk on water. By trying simple experiments, we can see that science is all around us—even in a glass of water.
References
- https://www.thoughtco.com/surface-tension-definition-and-experiments-2699204
- http://hyperphysics.phy-astr.gsu.edu/hbase/surten.html
- https://www.britannica.com/science/surface-tension
- https://www.usgs.gov/special-topics/water-science-school/science/surface-tension-and-water
