Wave-Particle
Duality Of Matter
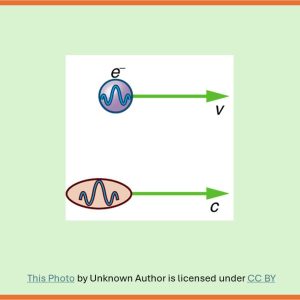
Overview
You may recall from the post ‘What exactly light is‘ that the quest to understand the nature of light eventually led to the discovery of wave-particle duality of light and also to a new field in physics: Quantum Mechanics.
Wave-particle duality of light signifies that light not only behaves like a wave but also like a particle.
After having learnt about the particle nature of light, Prince Louis de Broglie (1892 – 1987) of France wondered if light waves could act like particles, can particles like electrons behave like waves. Later, he theoretically proved that the answer to his question was an emphatic yes. For that discovery, he won the Nobel Prize for physics in 1929. However, the experimental verification for electron waves came from two other physicists who too won the Nobel Prize for their work.
Let us explore here how de Broglie expanded the idea of wave-particle duality of light to wave-particle duality of matter. First let us quickly review how the study of the nature of light proceeded over hundreds of years.
What exactly is Light?
Since ancient times, natural philosophers have been asking the question of what light really is. Up until the era of Newton, they believed that light consisted of particles. Newton called these light particles corpuscles. Unfortunately, the particle theory of light could not fully explain some of the phenomena of light such as refraction and diffraction though it could account for an array of light phenomena such as reflection and dispersion.
The Dutch physicist Christiaan Huygens (1629 – 1695) believed that light must be a wave. According to his wave theory, light waves travel through different materials by the propagation of wavefronts. Using the idea of wavefronts, Huygens was able to successfully explain all the known phenomena of light including diffraction.
In spite of the success of his theory, the particle theory of Newton was barely questioned for almost 150 years until the advent of the English physicist Thomas Young (1773 – 1829). The result of Young’s double slit experiment unequivocally established the fact that light must be a wave.
James Clerk Maxwell (1831- 1879) from Scotland unified electricity, magnetism and light. In that process, he not only predicted the existence of electromagnetic waves but also concluded that light must be electromagnetic in nature.
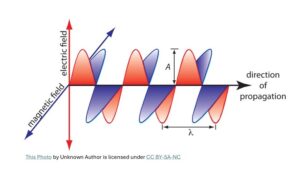
So, the wave theory of light was on a solid footing until physicists confronted strange puzzles after the study of blackbody radiation and the discovery of photoelectric effect. The wave theory of light alone could neither explain the photoelectric effect nor the blackbody radiation graph.
Wave-Particle Duality
of Light
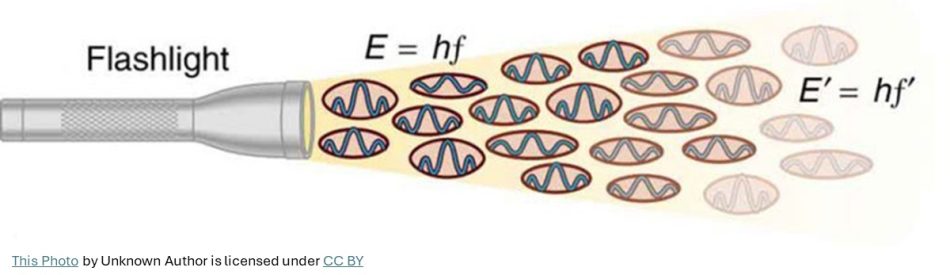
In 1900, in order account for the hill-like shape of the blackbody radiation graph (similar to the graph shown below), the German physicist Max Planck, as a desperate attempt, heuristically arrived at an equation.
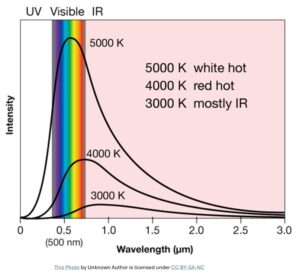
He reluctantly realized that he could explain the blackbody radiation only by introducing a tiny constant and thereby assuming that the light emission or absorption happens in discrete chunks (light-quanta.) That constant ‘h’ that he introduced in his equation is Planck constant which is now known as one of the fundamental constants of nature.
Though the equation perfectly matched with experimental data, Planck himself was not happy with his assumption of discrete nature of light to explain blackbody radiation results.
Then came the young genius Albert Einstein. He had a great insight that light was, indeed, not only a wave but also a particle. With that idea, he was able to successfully explain the photoelectric effect and discovered the following equation known as the law of photoelectric effect. He won the Nobel Prize in physics for the discovery of the law of photoelectric effect.
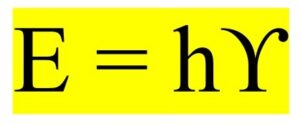
Amazing things happened after this discovery of particle nature of light. A new field in physics, Quantum Mechanics, was born. According to Quantum Mechanics, light is both a wave and a particle until you look at it in an experiment. This is known as the wave-particle duality of light.
Wave-Particle Duality
of Matter
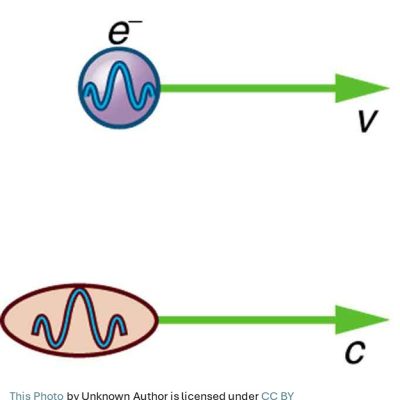
de Broglie’s matter waves:
As mentioned above, Albert Einstein, while explaining photoelectric effect, discovered that light was not only a wave but also a particle. On the other hand, Louis de Broglie (1892 – 1987) of France proposed that particles, such as electrons, were not only particles but also had waves associated with them.
When Louis de Broglie had learnt about the dual nature of light, he was convinced that both particle and wave theory of light made sense. Then, he wondered if light waves could act like particles, could particles like electrons behave like waves?
His doctoral dissertation work was on finding an answer to that question. He discovered that the answer to his question was an unequivocal yes.
By adopting Einstein’s photoelectric equation and the special theory of relativity, de Broglie theoretically derived the following equation. He showed that all particles, such as electrons, have associated waves and their wavelengths are given by the following equation.

His discovery confirmed the idea that the wave-particle duality of light be extended to encompass all matter. According to his theory, every particle, in fact all matter, has its own associated matter wave. For that remarkable discovery, de Broglie was awarded the Nobel Prize for physics in 1929.
However, the experimental confirmation of electrons waves came from Clinton Davisson (USA) and George Thomson (UK). For their work, together they won the Nobel Prize for physics in 1937.
Electron Microscope
Based on the above idea that electrons exhibit wave properties, two German scientists Max Knoll and Ernst Ruska invented the electron microscope in 1931.
Because electron’s wavelength is 100,000 times smaller than light waves, an electron microscope can produce images of much higher resolution than was possible with an optical microscope. In other words, the electron microscope has made it possible to observe immensely tiny things that was hitherto impossible to see with an optical microscope.
Key Takeaways
- Up until the time of Newton, natural philosophers thought that light consisted of particles. Newton called the light particles corpuscles.
- Particle theory of light could not explain all the phenomena of light.
- Christiaan Huygens proposed the wave theory of light. Later, Thomas Young, using his double-slit experiment results, confirmed that light was a wave.
- James Maxwell concluded that light is an electromagnetic wave.
- Max Planck and Albert Einstein introduced the light-quanta to explain blackbody radiation and photoelectric effect.
- According to quantum mechanics, light is both a wave and a particle.
- Louis de Broglie extended the idea of wave-particle duality of light to all the matter. He showed that particles like electrons have associated waves guiding them.
- Clinton Davisson and George Thomson experimentally confirmed the presence of electron waves.
- In 1931, exploiting the wave nature of electrons, German scientists Max Knoll and Ernst Ruska invented electron microscope.
References
- This Biggest Ideas in the Universe – Quanta and Fields: By Sean Carroll
- Quantum by Manjit Kumar
- Einstein by Walter Issacson
- https://youtu.be/2WPA1L9uJqo
- https://en.wikipedia.org/wiki/Matter_wave
