Heisenberg's
Uncertainty Principle

Overview
Heisenberg’s Uncertainty Principle, formulated by German theoretical physicist Werner Heisenberg in 1927, is a cornerstone of quantum mechanics. It posits a fundamental limit to the precision with which pairs of physical properties (of a quantum object), such as position and momentum, can be simultaneously known. Specifically, it asserts that the more accurately we measure a particle’s position, the less accurately we can measure its momentum, and vice versa.

It is crucial to understand that the limitations described by the principle are not due to measurement constraints but instead reflect the intrinsic nature of particles at the quantum level. This principle marked a significant departure from classical physics and has profound implications for our understanding of the quantum world.
To appreciate the full impact of Heisenberg’s insight, it is essential to explore the historical developments that paved the way for this groundbreaking principle.
The Dawn of
Quantum Theory
Planck’s Quantum Hypothesis (1900)
At the turn of the 20th century, classical physics was struggling to explain certain phenomena. For instance, classical physics failed to explain the hill-like shape (as the graph shown below) of black-body radiation. In order to account for this phenomenon, German physicist Max Planck, as a desperate attempt, heuristically arrived at an equation.
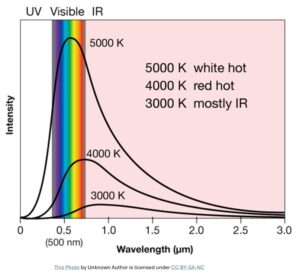
He reluctantly realized that he could explain the blackbody radiation only by introducing a tiny constant in his equation. The implication of that constant is that the light emission or absorption happens in discrete chunks (light-quanta.) That constant (h) that he introduced in his equation is now called Planck constant which is found to be one of the fundamental constants of nature. This quantum hypothesis laid the groundwork for the development of quantum theory.
Einstein’s Photoelectric Effect (1905)
Building on Planck’s hypothesis, Albert Einstein proposed that light itself is quantized and consists of particles called photons. In his 1905 paper on the photoelectric effect, Einstein demonstrated that light quanta could explain the emission of electrons from a metal surface when exposed to light. He determined that the energy of a photon can be represented by the following equation, where ‘E’ denotes energy, ‘h’ represents Planck’s constant, and ‘ϒ’ signifies the frequency of light.
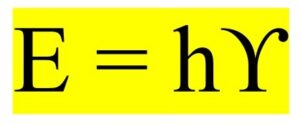
This remarkable discovery earned him the Nobel prize in physics in 1921 and further solidified the notion of quantization in physics.
Following Einstein’s discovery of the light photon, a pertinent question arose: if light can exhibit particle-like behavior, can particles such as electrons exhibit wave-like properties? The answer came from Louis de Broglie of France.
Wave-Particle Duality
De Broglie’s Hypothesis (1924)
In 1924, French physicist Louis de Broglie introduced the idea that particles can indeed exhibit wave like properties. De Broglie hypothesized that particles such as electrons have associated waves.
By adopting Einstein’s photoelectric equation and the special theory of relativity, de Broglie theoretically derived an equation for wavelength of a particle. He showed that all particles, such as electrons, have associated waves. He derived the following equation that gives the wavelength of the associated wave.

This discovery confirmed that the wave-particle duality of light could be extended to include all matter. According to his theory, every particle, in fact all matter, has its own associated matter wave. For that remarkable discovery, de Broglie received the Nobel prize in physics in 1929.
However, the experimental confirmation of electron-waves came from Clinton Davisson (USA) and George Thomson (UK). For their work, together they won the Nobel prize in physics in 1937.
Schrödinger’s
Wave Mechanics
Following the discovery of matter-waves by de Broglie and the experimental confirmation of their existence later, physicists started to wonder about the nature of matter-waves’ wave function. If there is a wave there should be a wave function to describe it. A wave function is a mathematical representation of quantum states, such as momentum, position and spin of a quantum system, over time.
In 1926, Austrian physicist Erwin Schrödinger formulated a theory that accurately described the wave function of quantum systems. This theoretical framework is referred to as wave mechanics. Schrödinger’s wave function has significant implications in the field of physics. The exploration of Schrödinger’s wave function and its ramifications will be addressed in a subsequent post.
Heisenberg’s Breakthrough
In 1927, Werner Heisenberg published a paper introducing the uncertainty principle, a significant and transformative contribution to quantum mechanics. This principle is one of the most renowned and perplexing aspects of the field.
The key insight of uncertainty principle is it is impossible to know the precise position of a particle, such as a moving electron, and its precise momentum at the same instant. The more precisely the position of the particle is measured, the less precisely it is possible to measure its momentum.
It underscores the intrinsic limitations of measuring quantum properties with absolute certainty. It has nothing to do with the limitations of measuring instruments or technology. In fact, the theory went beyond that. According to the uncertainty principle, an electron does not have a definite position or path until we observe it. This is the feature of the universe, not merely some defect in our observing or measuring abilities.
The following equation is the mathematical form of Heisenberg’s uncertainty principle.

Experimental Verification
The Uncertainty Principle has been experimentally verified through various methods, including the observation of electron diffraction patterns and the behavior of particles in quantum systems. These experiments have consistently demonstrated the inherent limitations in simultaneous measurements of complementary properties, validating Heisenberg’s theoretical predictions.
Impact on Physics
The Uncertainty Principle fundamentally altered our understanding of the physical world. It challenged the deterministic nature of classical physics and introduced the concept of probabilistic behavior at the quantum level.
Albert Einstein questioned the probabilistic nature of the quantum world. He supported the principle of causality. Consequently, Einstein argued that quantum physics was still incomplete.
Conclusion
The development of Heisenberg’s Uncertainty Principle was the culmination of decades of research and groundbreaking discoveries in quantum theory. From Planck’s quantum hypothesis to Einstein’s photoelectric effect, de Broglie’s wave-particle duality, and the formulation of matrix and wave mechanics, each step contributed to a deeper understanding of the quantum world.
Heisenberg’s Uncertainty Principle, encapsulated by the inequality Δx Δp ≥ ℏ/2, underscores the intrinsic limitations of measuring quantum properties with absolute certainty. This principle continues to shape our understanding of the quantum world, highlighting the intricate balance between knowledge and the inherent indeterminacy of nature.
References
- This Biggest Ideas in the Universe – Quanta and Fields: By Sean Carroll
- Quantum by Manjit Kumar
- https://youtu.be/MeK0DV329mU by Walter Lewin of MIT
- Einstein by Walter Isaacson.
