What Is Heat?

Overview
For centuries, one of the big questions that remained unresolved in physics was the simple question: what is heat? Physicists could not fully explain what gives rise to heat energy. Also, they could not understand why heat flows from hot to cold spontaneously but never does in the reverse direction.
Scientists of 18th and 19th centuries proposed several theories to explain heat energy. Only at the end of the 19th century, physicists could understand what it was.
Let us explore those theories and understand the modern theory of heat based on kinetic energy of atoms and molecules.
What is Heat?
The answer to this simple question remained a mystery till the beginning of 20th century. In 19th century, physicists (wrongly) believed that the heat was a result of the flow of an invisible fluid called ‘calorie.’

When water is heated, say by burning coal, physicists of those days believed that ‘calorie’ flowed from coal into water as the coal burnt. Even Sadi Carnot, the French physicist and engineer, published his steam engine theory, in 1824, based on the assumption that calorie existed, and that the calorie theory was correct.
It was James Joule, English physicist, who suspected the validity of calorie theory when he noticed that flowing electric current produced heat in wires. Also, he discovered that friction while drilling a metal object generated enormous heat. He wondered where calorie came from in those cases.
Though Joule pointed out that calorie theory could not be right, he too was not able to answer the question of what heat really was.
Bernoulli’s Theory
In 18th century, Daniel Bernoulli, Swiss mathematician and physicist, theorized that the kinetic energy from the random motion of the particles of fluids could be the reason for macroscopic attributes of fluids such as temperature and pressure. Since he was well advanced for his time, his ideas languished for almost hundred years.
Rudolf Clausius, German physicist and mathematician was the one who restarted the debate on the kinetic energy of gas particles in the year 1857, and he showed that kinetic energy from the random motion of the constituent particles of a gas could be responsible for the internal heat energy.
Scottish theoretical physicist James Maxwell, who believed in the atomic nature of matter, mathematically analyzed the kinetic energy of gases based on statistical methods.
In the next section, let us learn how James Maxwell and Boltzmann (Maxwell was Boltzmann’s hero) developed what is now known as kinetic theory of gases based on statistical methods.
Kinetic theory &
Statistical Mechanics


In 19th century, James Maxwell, who believed in the atomic nature, developed kinetic theory of gases using statistical method. Boltzmann extended Maxwell’s ideas in developing kinetic theory not only for gases but also for liquids and solids. They proved that the macroscopic properties of gases, liquids and solids are related to the average kinetic energy of their individual microscopic entities.
For instance, the temperature, pressure and internal energy of gas in a container are related to the average kinetic energy of the molecules of the gas. Kinetic energy arises from the random motion of the molecules either due to linear motion or from the spin of each molecule. The picture below illustrates the fact that the increased number of collisions on the wall, when the volume is reduced, is the reason for the increase of pressure.
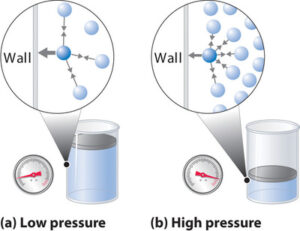
Since it is impossible to keep track of position, velocity and spin of each molecule of the gas in a container which may contain billions and billions of molecules, Maxwell and Boltzmann adopted statistical method to determine the average kinetic energy the gas.
The key conclusion from their works is that the energy that we feel as heat arises from the random motion (and collisions) of molecules in gases or liquids. In the case of solids, it is the vibration of the molecules or atoms we feel as heat.
Why does heat flow from hot to cold and not in the reverse? We will look into this question when we discuss the second law of thermodynamics in another post. You can read that post here.
Key takeaways
- Heat, a form of energy, arises in gases and liquids because of the random motion of its constituent molecules and random collisions. In solids, it is the vibration of their molecules or atoms that is the source of heat.
- The theory of kinetic energy of gases, based on statistical methods, proves that the macroscopic properties like temperature, pressure and internal energy are from the kinetic energy of its constituent molecules.
References
- Einstein’s Fridge by Paul Sen
- https://en.wikipedia.org/wiki/Ludwig_Boltzmann
- Britannica, The Editors of Encyclopaedia. “Ludwig Boltzmann”. Encyclopedia Britannica, 1 Sep. 2023, https://www.britannica.com/biography/Ludwig-Boltzmann. Accessed 6 February 2024.
- Britannica, The Editors of Encyclopaedia. “Statistical mechanics”. Encyclopedia Britannica, 15 Dec. 2022, https://www.britannica.com/science/statistical-mechanics. Accessed 10 February 2024.
- https://plato.stanford.edu/entries/statphys-Boltzmann/
