Why Heat Flows
From Hot
To Cold Side?

Overview
Why does heat energy spontaneously flow from the hot side to the cold side? Why does it never spontaneously flow in the reverse direction?
For instance, when you drop ice cubes in a glass of water, at room temperature, ice cubes start melting as heat energy flows from the water to the ice cubes. In this process, it lowers the temperature of water in the glass. The question is why heat energy does not flow from ice cubes into water?
Let us explore these questions and try to understand what the giants of heat energy say about them.
Heat Flow
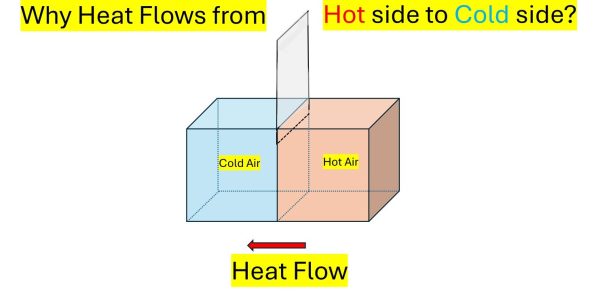
Now let us consider another example – a wooden box divided into two partitions separated by an airtight sliding door. Assume one side is filled with hot air and the other side is filled with cold air, as shown in the picture above.
What happens when you slide the door up to let the air from these two compartments mix? We know that the cold air gets warmer, and the hot air becomes cooler until the whole box reaches an equilibrium temperature. Obviously, thermal energy flows from the hot side to the cold side.
Again, the question is what stops heat energy from flowing from the cold side to the hot side?
Two Mysteries of Heat
For centuries, two questions, related to heat, remained mysteries.
The first question was: What really is heat. The works of James Joule, Rudolf Clausius, James Maxwell and Ludwig Boltzmann resulted in the full understanding of this simple but difficult question. This has been discussed in another post on our site: what is heat? You may read that post here to understand how they figured out the answer to this question.
The second question that remained another mystery was why heat energy always spontaneously flows from the hot side to the cold side. Why does it never flow from the cold side to the hot side spontaneously?
Only at the end of the 19th century, physicists could fully understand what heat was. However, the answer to the question – why heat flows from hot to cold – required a deeper insight into the laws of thermodynamics. Particularly, the second law of thermodynamics and its statistical reasoning ultimately provided the answer to this question.
Laws of Thermodynamics
Though there are four laws of thermodynamics, two laws are particularly important in answering our question – The first and second law of thermodynamics.
As you know, the first law of thermodynamics deals with the conservation of energy. When energy exchange happens between two systems, the total energy before and after the energy exchange remains the same.
And the second law of thermodynamics states that entropy (the degree of randomness) of a closed system increases, and it can never go down on its own. (If you would like to learn more on entropy please read this post.)
Now, let us explore how kinetic energy gases, statistical mechanics, and the second law of thermodynamics, explain why heat energy flows from the hot side to the cold side and not in the reverse.
The Answer:
Statistical Probability
According to the second law of thermodynamics, the entropy of a closed system tends to increase. It was Boltzmann who provided the correct definition of entropy. He also explained why the second law of thermodynamics was true. His definition of entropy was based on the number of arrangements of atoms or molecules in a system. He showed that increasing entropy was natural result of the fact that increasing entropy was statistically more probable than decreasing entropy.
The same argument holds for why heat energy flows from the hot to the cold side. This happens because flowing from hot to cold is highly statistically probable than flowing in the reverse direction. Though the flow in the reverse direction is very much plausible, the extremely low probability of this thing happening makes it not occurring in reality.
Just to illustrate this point, let us consider an example. For instance, think of a deck of shuffled playing cards in its pristine rank-and-suit precise order.

As you shuffle the deck, does randomness increase or decrease? Will the deck ever end up back in its pristine rank-and-suit precise order as you shuffle the deck repeatedly? It is extremely unlikely. The probability that it will end up that way is extremely low, whereas the probability of getting increasingly random is extremely high.
The point is, as you shuffle more, what is happening is that the randomness of the deck increases because it is (statistically) highly probable than ending up in a pristine state. That is the reason you will never see the pristine state even if you shuffle the deck whole of your lifetime!
Conclusion
In conclusion, when heat exchange occurs between two systems as they come in contact, heat energy spontaneously can flow from the hot side to the cold side because it is statistically more probable.
Hot systems have atoms and molecules with higher average kinetic energy than the cold systems. So, higher kinetic energy particles imparting kinetic energy to lower kinetic energy particles is more likely to happen than the other way around.
References
- Einstein’s Fridge by Paul Sen
- https://en.wikipedia.org/wiki/Ludwig_Boltzmann
- Britannica, The Editors of Encyclopaedia. “Ludwig Boltzmann”. Encyclopedia Britannica, 1 Sep. 2023, https://www.britannica.com/biography/Ludwig-Boltzmann. Accessed 6 February 2024.
- Britannica, The Editors of Encyclopaedia. “Statistical mechanics”. Encyclopedia Britannica, 15 Dec. 2022, https://www.britannica.com/science/statistical-mechanics. Accessed 10 February 2024.
- https://plato.stanford.edu/entries/statphys-Boltzmann/
